July 24, 2020 feature
Designing a freestanding, supercharged polypeptide proton-conducting membrane

Thamarasee Jeewandara
contributing writer
Protons are subatomic particles with a positive electric charge. plays a significant role and . But it remains challenging to control conduction and fabrication in biomaterials and devices. In a new report, Chao Ma and an interdisciplinary team of scientists in China, the Netherlands, and Germany, rationally designed proton-conducting protein constituent materials that exceeded previously reported proteinaceous (consisting of or containing protein) systems. They developed the structures through stepwise exploration of from intrinsically disordered coils to protein-supercharged polypeptide chimeras. The new design paradigm offers potential for device fabrication at the interfaces of artificial and biological systems, the results are published on Science Advances.
Proton conduction is responsible for fundamental processes in biology, including , the synthesis of (ATP) and . Bioengineers and materials scientists had previously developed several synthetic materials with proton translocation behavior including , although their shortcomings have impeded the fields of bioelectronics and biotechnology. To develop biomaterials dedicated for proton conduction, scientists must explore scaffolds and sequences for their . During hydrated states, protons can be transported via water molecules along an adjacent bond network in a mechanism known as proton hopping, which is used as a blueprint to design proton-conducting structures de novo (i.e. from scratch). In this work, Ma et al. developed a stepwise, protein-based proton-conducting membrane with a set of unfolded, anionic (SUPs) containing residues.
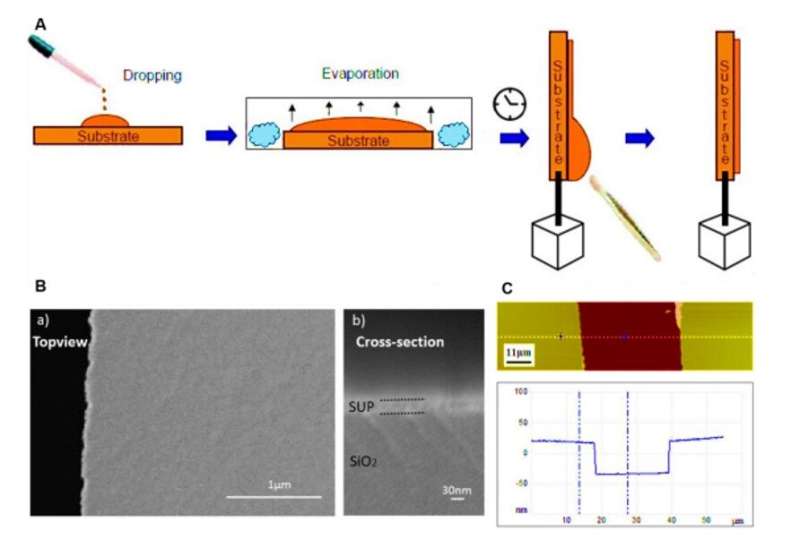
Developing proton-conducting protein materials
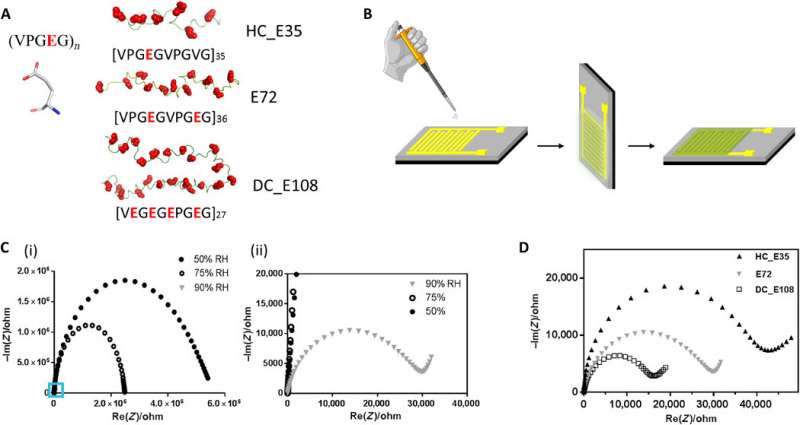
In the backbone of the proton-conducting membrane, the hydrophilic (water-loving) charged moieties served as proton carriers. The team studied the proton-conducting performance of these unfolded systems to obtain freestanding membranes and perfected the structural design by amalgamating silk-like with anionic SUPs to form self-assembled nanostructures. The team decorated the surfaces with dense groups for hydration, proton dissociation and to form proton conduction pathways. The mechanically stable and freestanding membrane surpassed hitherto reported transport properties of protein-based systems for outstanding proton conductivity.
The team derived the supercharged proteins from ; for applications of and interface modification. They introduced (abbreviated Glu or E), which can be easily deprotonated under physiological conditions into the X site of the protein sequence, to form unstructured negatively supercharged polypeptides (SUP-Es). Then they constructed three different variants of supercharged polypeptides known as E72, HC_E35 and DC_E108. Ma et al. used (EIS) with gold interdigitated electrodes (IDEs) to evaluate thin-film proton conduction and measured proton transport as a function of relative humidity. When the humidity increased to 90 percent, proton translocation improved due to absorption of a large number of water molecules via the carboxylic acid (-COOH) groups of the material. Besides relative humidity, they also investigated proton conduction relative to for the specimens of interest. By tuning the charge density of the disordered proteins, Ma et al. successfully controlled proton conductance behavior of proteins within films. Due to the high stability and uniformity of the thin films made of SUPs, the setup did not show signs of defects.
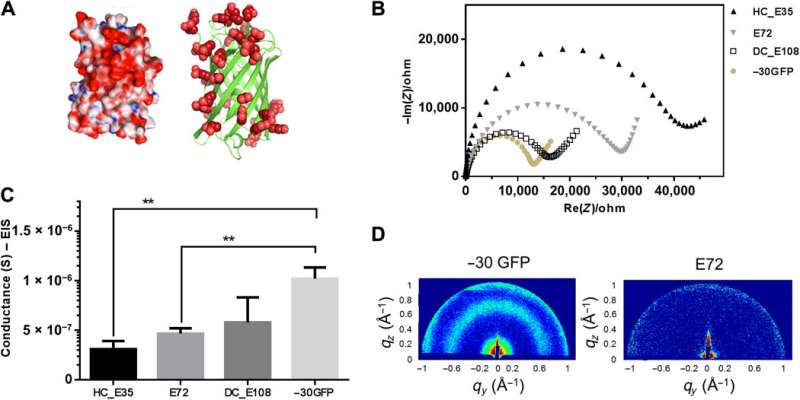
Folded protein backbones and spider-E development
Ma et al. then further studied folded nanosized protein backbones and equipped the nanoscale scaffolds with carboxylic acid on the surface—similar to SUPs. Using , they investigated structural information inside the supercharged protein samples to obtain distinct signatures of their structural domains, to show how nanostructured components could facilitate proton translocation. The work allowed the team to rationally engineer protein motifs to perform proton conduction. Motivated by increased proton conductivity, Ma et al. combined the resulting design elements with the existing supercharged polypeptide (SUP) structures.
Instead of using β-barrel motifs in the materials architecture, they used the mechanically stable β-sheet structures—a sequence . They named the combined system of anionic SUP with β-sheet sequences as 'spider-E'. The scientists produced the anionic spider-E material using systems in the lab and determined the structure using X-ray diffraction, (FTIR) spectroscopy and . The spider-E film showed higher proton conductance compared to amorphous SUP films alone.
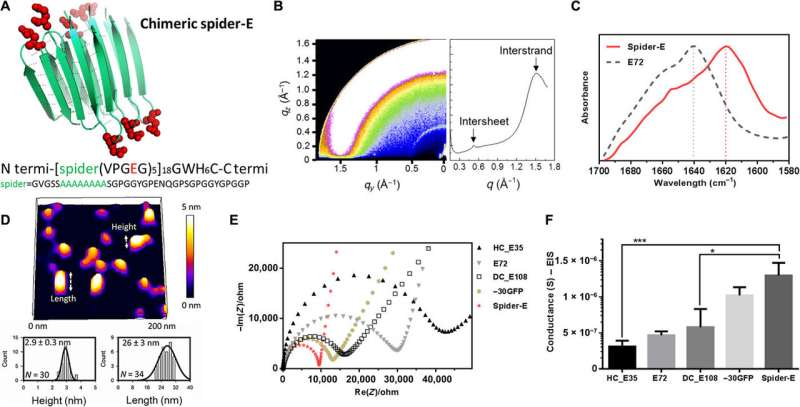
Characterizing the spider-E material architecture
The β-sheet structured material system showed improved mechanical properties as a free-standing membrane that could be easily produced. For instance, Ma et al. drop casted the spider-E solution to engineer a transparent macroscopic membrane in the lab. The results showed mechanical robustness of the construct due to the inclusion of spider motifs with a yield strength comparable to . The researchers showed how spider motifs formed β-sheet structured domains with hydrophilic surfaces composed of glutamic acid-rich SUP strands, to facilitate excellent proton hopping. The study pushed the limits of existing proteinaceous proton-conducting materials to represent a key example of protein engineering. The work represents one of the first examples that combines protein engineering and the rational design of bulk architecture with collective properties from molecular ensembles.
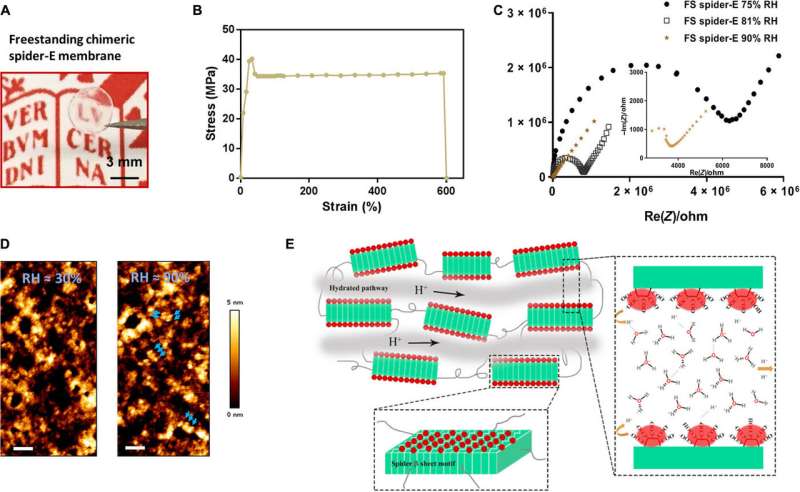
In this way, Chao Ma and colleagues applied rational molecular de novo design and engineering to achieve a bioinspired protein-derived bulk material with robust properties of proton conduction and excellent mechanical stability. They tested the surface modifications using a range of biophysical tools. The team developed the new generation, bioinspired bulk material and explored successive sequence designs to offer a promising platform for applications in biotechnology and envision the use of such materials for proton transport in miniaturized of the future.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Chao Ma et al. De novo rational design of a freestanding, supercharged polypeptide, proton-conducting membrane, Science Advances (2020).
I. N. Watt et al. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria, Proceedings of the National Academy of Sciences (2010).
Jeff A. Hurd et al. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework, Nature Chemistry (2009).
Journal information: Science Advances , Proceedings of the National Academy of Sciences , Nature Chemistry
© 2020 Science X Network